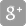SI-BONE, Inc. Receives FDA 510(k) Clearance for a Smaller Diameter iFuse Bedrock Granite Implant with an Expanded Indication and Application
SANTA CLARA, Calif., Jan. 30, 2024 (GLOBE NEWSWIRE) -- SI-BONE, Inc., (Nasdaq: SIBN), a Silicon Valley-based medical device company dedicated to solving musculoskeletal disorders of the sacropelvic anatomy, announces FDA 510(k) premarket clearance of the iFuse Bedrock Granite® Implant System (Granite) in a smaller (9.5 mm) diameter with both an expanded indication in pediatric patients and an expanded application that includes use in the S1 trajectory. When placed across the SI joint, the Granite implant provides sacroiliac fusion and sacropelvic fixation as a foundational element for multi-segment spinal fusion.
This 510(k) clearance follows the initial clearance of the iFuse Bedrock Granite System in May 2022 that included implants of 10.5 mm and 11.5 mm in diameter. The iFuse Bedrock Granite System was also awarded a Breakthrough Device Designation (BDD) by the Food & Drug Administration (FDA) and a New Technology Add-on Payment (NTAP) by the Centers for Medicare and Medicaid Services (CMS).
According to Chris Shaffrey, MD, Chief of the Spine Division at Duke University, “The addition of a 9.5 mm diameter implant will seamlessly blend into my workflow, especially with both closed head and open head options. I’m very excited for patients to benefit from this breakthrough spinopelvic technology.”
“With the expanded sacral indication and a smaller diameter Granite implant, I can utilize the Granite technology at S1, a segment which published literature has shown to be one of the most challenging segments in the spine, with reported screw loosening rates ranging from 16-41%,”1,2,3 said Brian A. O’Shaughnessy, MD, Reconstructive Spinal Surgeon at Howell Allen Clinic in Nashville, TN.
Greg Mundis, MD, Orthopedic Spine Surgeon at Scripps Hospital mentions, “There is increasing interest among the surgeon community to include pelvic fixation in high-risk patients undergoing shorter (2-3 level) lumbar fusions. The addition of a 9.5 mm diameter to the Granite product line now offers surgeons one of the more commonly used sizes to treat these patients.”
About SI-BONE, Inc.
SI-BONE (NASDAQ: SIBN)?is a global leader in technology for surgical treatment of musculoskeletal disorders of the sacropelvic anatomy. Since pioneering minimally invasive surgery of the SI joint in 2009, SI-BONE has supported over 3,600 surgeons in performing a total of more than 95,000 sacropelvic procedures. A unique body of clinical evidence supports the use of SI-BONE’s technologies, including two randomized controlled trials and over 125 peer reviewed publications. SI-BONE has leveraged its leadership in minimally invasive SI joint fusion to commercialize novel solutions for adjacent markets, including adult deformity, spinopelvic fixation, and pelvic trauma.
For additional information on the company or the products including risks and benefits, please visit www.si-bone.com.
iFuse Bedrock Granite, iFuse-TORQ and SI-BONE are registered trademarks of SI-BONE, Inc. ©2024 SI-BONE, Inc. All Rights Reserved.
Investor Contact: Saqib Iqbal investors@si-bone.com
References
- Xu F, Zhou S, Zou D, Li W, Sun Z, Jiang S. The relationship between S1 screw loosening and postoperative outcome in patients with degenerative lumbar scoliosis. BMC Musculoskelet Disord. 2022 Feb 28;23(1):186.
- Kim JB, Park SW, Lee YS, et al. The effects of spinopelvic parameters and Paraspinal muscle degeneration on S1 screw loosening. J Korean Neurosurg Soc. 2015;58(4):357–362.
- Finger T, Bayerl S, Onken J, et al. Sacropelvic fixation versus fusion to the sacrum for spondylodesis in multilevel degenerative spine disease. Eur Spine J. 2014;23(5):1013–1020.
A photo accompanying this announcement is available at:
https://www.globenewswire.com/NewsRoom/AttachmentNg/d881faa0-5a12-49b0-8f66-1d802e65cebd


Upcoming Life Sciences Events
- May 2024
- London: London BioTechnology Show 2024
- Seoul: BIO KOREA 2024
- Biotechgate Digital Partnering May 2024
 Biotechgate Global Database ›
Biotechgate Global Database ›