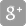Overview
Typical activities
- Identification of therapeutic need
- Target research
- Strategies for product development
- Competitor analysis
- Market analysis
- Target Product Profile (TPP)
Typical activities
- Target Product profile (TPP)
- Target validation studies
- Primary assay
- Preparation of targets
- Biomarkers
- In Silico-models
Typical activities
- Supply of substance libraries
- Screening of molecules
- Evaluation and assessment of potential lead molecules
Identification, development an optimization of molecules with higher potency and selectivity
Typical activities
- Pharmaceutical chemistry (synthesis and SAR-analysis)
- Drug ability-tests(ex. ADME-analyse)
- Target selectivity
- Questions regarding intellectual property
- SCREENING AGAINS SAFETY PANELS
- Effect and exposure analysis- Assay screening
- Molecular design (SAR-analysis)
- Formulation for animal studies
- In vivo animal sudies, pharmacodynamics (PD)
- In vivo animal studies, pharmacokinetics (PD)
- In vivo tolerance and effacacy studies
- In vivo-tox
Development and optimization of the production process for biomolecules
Typical activities
- Cell line development
- Cell culture
- Stability studies
- Production of active substance ("upstream development") and scale up
- Pre-formulation studies
- Analytical development (e.g. fucntional assays
Optimization and validation of the production process for biomolecules
Typical activities
- "Downstream development"
- Manufacture of MCB (Master Cell Banks)
- Manufacture of WCB (Working Cell Banks), cGMP
- Virus validation and process characterization
- Qualification and validation of analytical studies
- Manufacture and release of cGMP material for clinical studies
- Stability studies
- Regulatory support
- Regulatory strategy
Typical activities
- Regulatory support
- Regulatory strategy
- Pre-formulation
- CMC (according to cGMP)
- Production of active substance
- API-scale up
- Analytical development
- Pharmaceutical development
- Toxicology and safety (in vivo)
- Non-GLP
- GLP (based on clinical plan)
Typical activities
- IMPD compiling
- Development, planning and design of clinical studies incl. regulatory valid endpoints
- Facilities for clinnical studies
- Regulatory support
- Regulatory strategy
- Non-clinical studies (carcinogenicity, reproductive and developmental toxicity) to be considered during clinical development
Development of API-production and formulations
Typical activities
- Formulation development
- Manufacturing Process development
- Analytical method development
- Drug substance (API) scale up
- Drug product (formulation) scale up
- Stability trials for shelf-life
- GMP
- Quality risk management
- Site master file preparation
- Audits and inspections
- Biologics: validation for commercial production
Typical activities
- Phase 0 – Micro dosing
- First in man: Healthy volunteers
- Assess dose tolerance
- Single and multiple dose PK and/or PD
- Explore drug metabolism (ADME) and drug interactions
- Bioanalytical development
- Side effects
- Benefit/risk ratio
- Regulatory support
- Regulatory strategy
Clinical studies for proven safety and efficacy
Therapeutic exploratory studies
Therapeutic exploratory studies
Typical activities
- Small scale patient studies
- Explore safety and efficacy for targeted indication
- Explore dose-response relationship
- Benefit/risk ratio
- Bioanalysis validation
- Basis for Phase 3 trial design, incl statistical analysis plan
- Paediatric investigation plan (PIP) agreement with EMA (PDCO)
- Regulatory support
- Regulatory strategy
- Health economy, cost efficiency
Clinical studies for proven safety and efficacy
Therapeutic confirmatory studies
Therapeutic confirmatory studies
Typical activities
- Large scale patient studies
- Confirm efficacy
- Establish safety profile
- Establish dose-response relationship
- Assessment of benefit/risk ratio to support licensing
Documentation and applications for approved sale and use of a drug, as well as reimbursement of pharmaceuticals
Typical activities
- Market authorisation application (MAA)
- Application support for NDA submission
- Product launch approval from official authorities
- Reimbursement applications at TLV etc.
Typical activities
- Production
- Product launch
- Market analysis
- Marketing
- Sales
- Distribution
Typical activities
- Side-effect reporting (pharmacovigilance) for less common adverse events
- Long term effects of treatment
- Safety and risk management
- Approval for paediatric use
- Withdrawals
- Variations to MA
- Line extensions (new indications, new pharmaceutical forms etc)
- Price changes
- Expansion to new markets
- PASS studies and other post-approval commitments
Upcoming Life Sciences Events
- April 2024
- Basel: Swiss Biotech Day 2024
- London: BioTrinity 2024
- Singapore: Asia Bio Partnering Forum
 Biotechgate Global Database ›
Biotechgate Global Database ›